Our vision is to maximise cancer trial access for Irish patients. To achieve this we want to see the number of cancer trials opening in this country go from strength to strength. We have a rich history of conducting practice-changing clinical trials and publishing clinical trial results – you can see some of our trial success stories at the bottom of this page.
If you are a Cancer Trials Ireland member interested in opening a clinical trial, we can help.
Please see the pathways below for each trial type. If you are unsure where your trial fits, or have questions, you can contact info@cancertrials.ie
Investigator Initiated Trials (IIT)
Clinical trials involving investigational drugs require a sponsor to manage legal, financial, and insurance obligations. Cancer Trials Ireland (CTI) is the most experienced academic sponsor in Ireland, providing expert support to investigators throughout the trial process. CTI can sponsor your IIT.
Our IIT Pathway
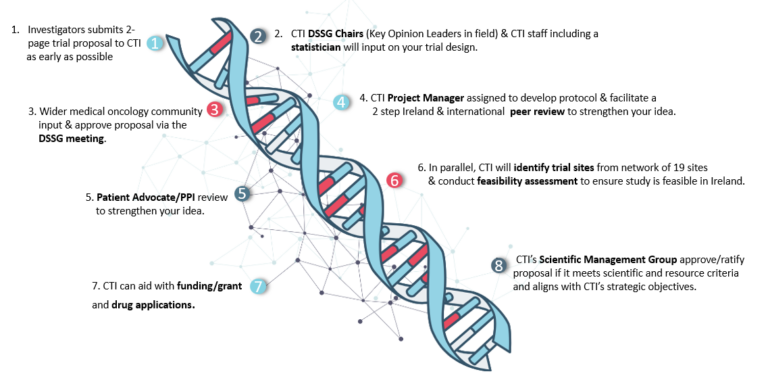
- Submit your trial concept to CTI as early as possible using this form.
- CTI DSSG Chairs (key opinion leaders) and our experienced team, including statisticians, will provide expert guidance on trial design.
- Your proposal will be discussed within DSSG meetings, ensuring input from the wider oncology community.
- CTI facilitates peer reviews and Patient and Public Involvement (PPI) feedback, strengthening your trial’s impact.
- Your study will be assessed by the Scientific Management Group (SMG) for scientific merit, resource feasibility, and alignment with CTI’s strategic goals.
- CTI helps identify suitable trial sites within our network of 19 research centres and conducts feasibility assessments to ensure successful implementation.
- CTI provides guidance on funding applications, grant submissions, and drug access to support your trial’s success.
By partnering with Cancer Trials Ireland, you gain access to a trusted academic sponsor with extensive expertise in managing investigator-led clinical trials.
International Collaborative Trials
Cancer Trials Ireland has a proven track record of working with international research groups such as ECOG, NRG, and ANZUP to bring global clinical trials to Ireland. We facilitate the opening of international collaborative trials and can act as the EU sponsor for non-EU studies where required.
Collaborative Trials Pathway
- Submit the trial concept or protocol to the relevant Disease Specific Sub-Group (DSSG) as early as possible.
- DSSG Chairs (key opinion leaders in the field) assess the trial’s suitability for Ireland.
- The trial is presented at a DSSG meeting, where the wider oncology community reviews and approves its opening in Ireland.
- The study is reviewed by Cancer Trials Ireland’s Scientific Management Group (SMG) for scientific merit, resource feasibility, and alignment with CTI’s strategic goals.
- We help identify suitable trial sites within our network of 19 research centres and conduct feasibility assessments to support successful implementation.
- Cancer Trials Ireland can assist with funding and grant applications if required.
- We work closely with the international sponsor to facilitate trial opening in Ireland and can take on the EU sponsor role where needed.
By leveraging our expertise and established international collaborations, Cancer Trials Ireland makes it easier to bring innovative global trials to Irish patients.
Adopted Trials
An adopted trial is a clinical trial managed by an external sponsor, such as the pharmaceutical industry or academia. Adding your trial to the Disease Specific Sub-Group (DSSG) portfolio can increase its visibility within the Irish medical oncology community, encouraging patient accrual and clinician referrals.
Cancer Trials Ireland provides a feasibility service for industry trials – submit a request or inquiry by email at feasibilities@cancertrials.ie.
Benefits of Adding Your Trial to the DSSG Portfolio
- Increased Exposure: Your trial will be included in the DSSG Portfolio Map and discussed at regular DSSG Investigator Meetings, ensuring awareness among key stakeholders.
- Enhanced Communications: Updates on your study will be featured in Cancer Trials Ireland (CTI) and DSSG newsletters, which reach CTI members, including 98% of cancer-treating specialists in Ireland.
- Public & Professional Access: Once open to recruitment, your trial will be listed in the Open Trials section of the CTI website—a key resource for both medical professionals and the public.
- ONCOassist Integration: Your trial will also be featured on the ONCOassist app, a widely used mobile platform for oncology professionals. Cancer Trials Ireland supports ONCOassist by maintaining a current listing of open cancer trials, helping clinicians identify suitable trials for their patients.
Adopted Trials Pathway
- Submit the concept or protocol to the relevant DSSG as early as possible.
- The study will be assigned a CTRIAL-IE Number and added to the portfolio immediately. It will also be included in future DSSG meetings and communications.
- Site Feasibility: Cancer Trials Ireland can assist in identifying suitable trial sites within our network of 19 sites and conduct feasibility assessments to ensure the study is viable in Ireland.
- Once the trial is open to recruitment, it will be added to the CTI website and ONCOassist app for increased visibility.
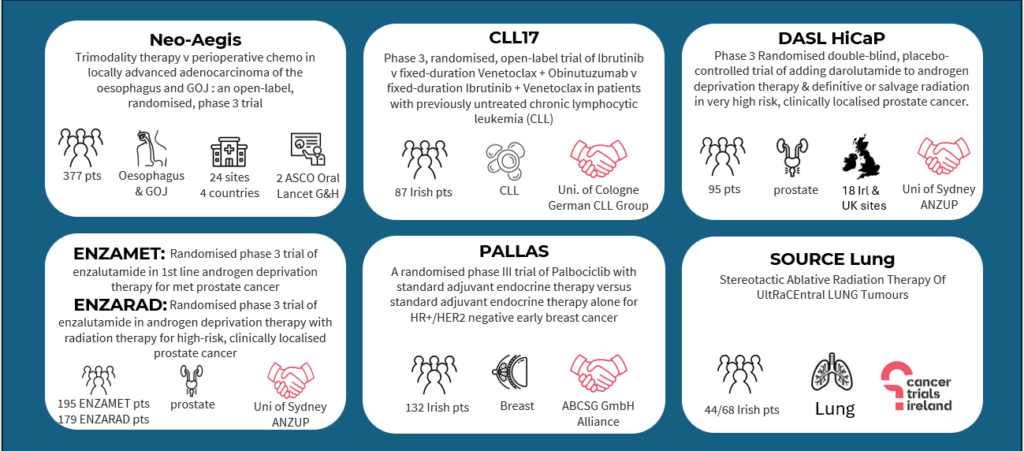
Trial Success Stories